FDA warns public vs. fake paracetamol tablets
The Food and Drug Administration (FDA) has advised the public and healthcare professionals against buying and using fake paracetamol tablets.
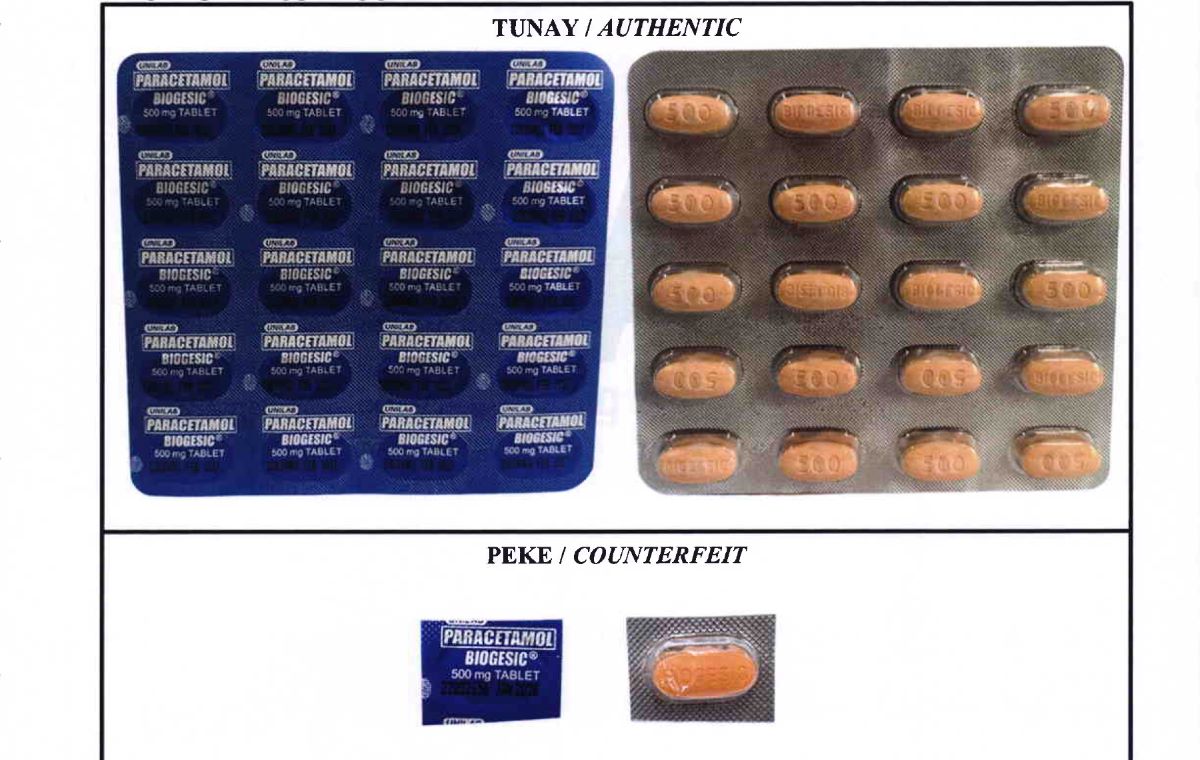
In an advisory signed by FDA Director General Samuel Zacate, the agency showed photos of a counterfeit paracetamol (Biogesic®) 500 mg tablet that has a different lot number, capsule, knurling, and print appearance as compared to the authentic one.
The FDA thus reminded the public to only purchase medicines from FDA-licensed establishments, as fake ones may pose health risks to those who would take them.
The agency also warned all establishments not to sell fake medicines, as they would be held legally liable.
“Ang pagaangkat, pagbebenta at pamamahagi nito ay paglabag sa Republic Act No. 9711 or the Food and Drug Administration Act of 2009, and Republic Act No. 8203 or the Special Law on Counterfeit Drugs. Ang sino mang mapatunayang nagbebenta ng nasabing pekeng produkto ay mapaparusahan,” the advisory read.
(Its importation, sale, and distribution are violations of Republic Act No. 9711 or the Food and Drug Administration Act of 2009, and Republic Act No. 8203 or the Special Law on Counterfeit Drugs. Whoever is found to be selling this fake product will be punished.)
The FDA also urged local government units and law enforcement agencies to ensure that the fake product would not be sold or used in their respective jurisdictions. —VBL, GMA Integrated News




