FDA warns vs. fake versions of Sanofi Pasteur’s anti-rabies vaccine for human use
The Food and Drug Administration (FDA) on Monday warned the public, especially healthcare professionals, against fake anti-rabies vaccines for human use circulating in the market as these pose potential dangers to consumers.
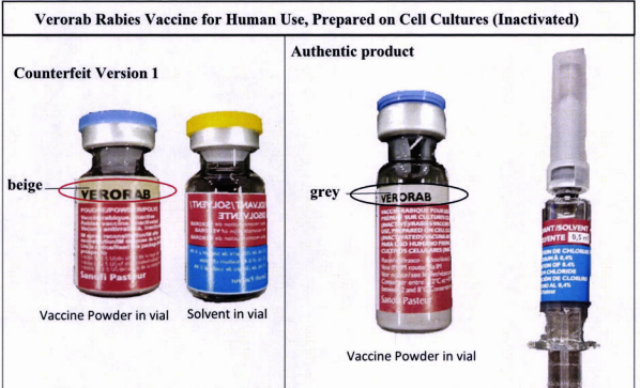
According to FDA advisory 2019-190, the original Verorab Rabies Vaccine for Human Use, Prepared on Cell Cultures (Inactivated) has been counterfeited.
Sanofi Pasteur Inc. manufactures the authentic product.
Authorities have been able to pinpoint five counterfeit versions through a verification process.
The public is advised against buying the anti-rabies vaccine with the following lot numbers:
- Lot no. H1742 (Counterfeit version 1)
- Lot no. H1833 (Counterfeit version 2 and 3)
- Lot no. N1E353M (Counterfeit version 4)
- Lot no. N1J75V (Counterfeit version 5)
Among the discrepancies observed in the fake products' packaging were wrong registration numbers, larger font sizes and different font style, and lack of stick-on label and translation of product details in two other languages than English.
The FDA did not specify the manufacturer of counterfeit versions and the distributors of Verorab in the Philippine market.
Local authorities and the Bureau of Customs were enjoined to prevent the entry and distribution of the fake Verorab vaccines.
The FDA said those who will be caught selling or dispensing the verified counterfeit products may face sanctions under the Food and Drug Administration Act of 2009 and Republic Act No. 8203 or the Special Law on Counterfeit Drugs.
It also advised the public to purchase drug products only from FDA-licensed establishments. —VDS, GMA News




